Lipiodol Fallopian Tube Flush Procedure
What is Lipiodal Tubal Flushing?
Lipiodol is a liquid contrast agent made from poppy seed oil. It was originally found to be useful because it contains iodine which makes it radio-opaque. However, we no longer need to use x-rays, but use ultrasound to follow its flow. To make the Lipiodol visible with ultrasound, we aerate it before its instillation.
Recent studies have shown that Lipiodal tubal flushing improves the chances of pregnancy.
The Ultrasound Care team of specialist obstetricians and sonographers have performed Lipiodol tubal flushing procedures on hundreds of women who have had difficulty becoming pregnant.

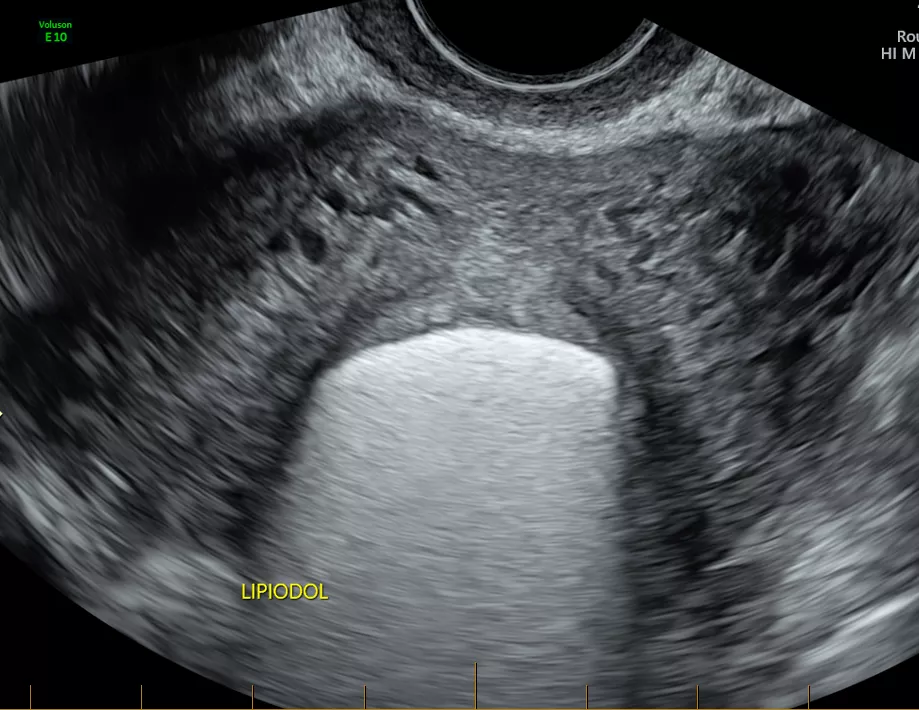
The white in the centre of the image is Lipiodol within the uterine cavity.
How is the procedure done?
Ultrasound Care uses ultrasound guidance to avoid radiation exposure. When you come for your procedure, your doctor inserts a sterile speculum into the vagina and the cervix is washed with antiseptic. A soft narrow plastic tube is then inserted into the uterus so that the Lipiodol can be introduced into the uterus and Fallopian tubes. With a simultaneous trans-vaginal ultrasound examination being conducted by the sonographers, the Lipiodol can be visualised passing into the uterus, out both Fallopian tubes and spilling into the pelvis. This tubal flushing appears to improve the chances of pregnancy in the next 6 months after the procedure.
How does it improve the chances of pregnancy?
In recent papers, the Lipiodol flush procedure was shown to improve the chances of pregnancy. The exact mechanism has not yet been confirmed, but it is believed that the Lipiodol may:
- Help embryos implant into the endometrium.
- Clear out the Fallopian tubes.
- Alter the balance of macrophage and other immunological cell activity around the tubes and ovaries.
Who is not suitable for the procedure?
Women with an Iodine allergy
As Lipiodol contains Iodine, it is not suitable for women who have a history of Iodine allergy.
NB: Iodine may also interfere with the function of the thyroid gland. If you have a thyroid problem, you should discuss the risk with your doctor first.
Women who are already pregnant or could have conceived this cycle
If you believe you may have conceived or are already pregnant, Lipiodol flushing should not be performed as we do not want to disturb the pregnancy.
To ensure that you are not pregnant on the day of the procedure, our care team will usually book an appointment date before day 12 of your cycle, but after bleeding has ceased.
Women with blocked tubes
Lipiodol flushing is only appropriate for women who have functioning Fallopian tubes as we do not want to intentionally inject the Lipiodol and have it collecting in blocked tubes as this increases the chances of complications.
Ultrasound Care therefore recommend that tubal patency be confirmed before a Lipiodol flushing procedure. If you have had a previous tubal patency test, please bring a copy of your previous hystero-salpingogram (HSG), hystero-contrast-sonography (HyCoSy) or laparoscopy and dye report to your appointment.
If tubal patency has not been assessed previously, it can be done at the time of the Lipiodol flushing, with air aerated saline. The disadvantages of this approach means that the whole procedure takes longer and maybe harder for you to tolerate. There is also is no good evidence about whether flushing with saline before the Lipiodol affects the results or the pregnancy outcomes.
Women with a risk of pelvic infection
Neither Lipiodol tubal flushing or HyCoSy should be performed in women with suspected pelvic infection. If you have a concern about a past history of pelvic infection, please discuss the option of prophylactic antibiotics with your referring doctor before booking your procedure.
What are the risks and complications?
Lipiodol tubal flushing is a minimally invasive procedure that is well tolerated and is associated with a very low risk of side effects.
The known risks of the Lipiodol flushing are:
- Allergic reaction to Iodine - This is more common if there are blocked tubes and high pressures are used during the procedure which could result in Lipiodol entering blood vessels of the uterus directly.
- Lipiodol can persist in the pelvic cavity for several weeks. There do not appear to be any harmful effects arising from this. It is eventually absorbed.
- Rarely the procedure may activate pelvic inflammation.
- Granuloma formation - This is a chronic inflammatory response to Lipiodol and, in extremely rare circumstances, could lead to the need for surgery. An example would be to remove an inflamed Fallopian tube affected by a granuloma.

Is Lipiodol tubal flushing invasive and what is its success?
Lipiodol tubal flushing has been performed in different ways for decades. The techniques have been perfected, and it has now become a very well tolerated, minimally invasive procedure.
A recent meta-analysis of multiple trials where lipiodol has been used with x-ray, has shown higher pregnancy rates in the 6 months post-procedure for those couples who had the Lipiodol flush procedure. The articles show that not all patients conceived after the procedure. It does not guarantee a pregnancy for everyone as there are many other important factors relating to a woman’s ability to conceive.
How do I find out if Lipiodol tubal flushing is right for me and then arrange for the procedure?
You should discuss the risks and benefits of the Lipiodol flush procedure with your fertility specialist to understand if the procedure is suitable for you. If your doctor believes you are a suitable candidate for Lipiodol tubal flushing, please arrange for a referral and call an Ultrasound Care practice that is convenient to you in Sydney to arrange for an appointment.